1. Urea or Lime Can Reduce Overwintering Inoculum of Apple Scab and Marssonina Leaf Blotch in Apple Leaf Litter, 2. Peach Leaf Curl Insurance with Ziram, Copper (Fall) and Chlororthalonil (Spring)
I. APPLE SCAB (Venturia inaequalis)
If symptoms of apple scab were visible on leaves or fruit during the 2023 growing season, you should plan to reduce overwintering scab inoculum residing in leaf litter on the orchard floor. What you do now for inoculum reduction can increase the efficacy of your apple scab fungicides in 2024 growing season. The most conducive for apple scab fungus overwintering are numerous late summer and fall infections on leaves visible as small lesions on the underside (Figure 1).

The late season/fall scab lesions are the most productive places where apple scab fungus Venturia inaequalis will grow into the internal tissue of leaves after they reach the orchard floor and form initials of pear-like fruiting bodies of the fungus called pseudothecia (Figure 2). These pseudothecia will facilitate release of apple scab ascospores in the spring of 2024 with each wetting event, starting from green tip on apples.
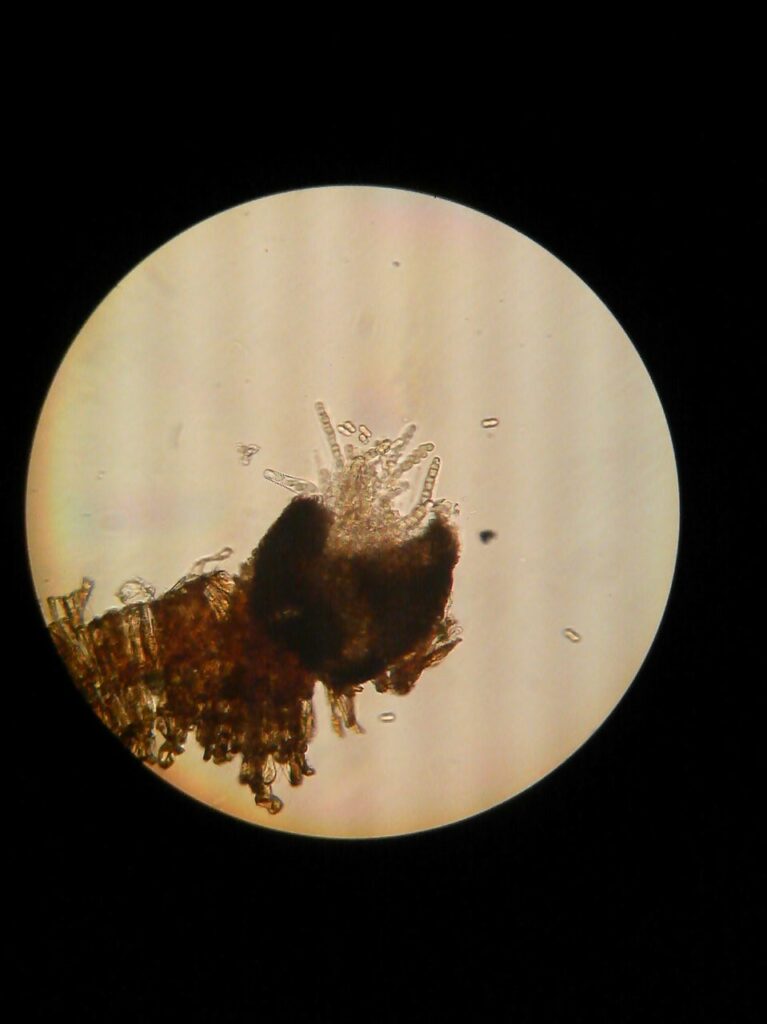
Pseudothecia are protected inside the dead apple leaves on the orchard floor and when they mature they release ascospores (Figure 3) which cause first infections on green apple bud tissues in the spring (leaves, fruit and petioles of both leaves and fruit). If you had a high disease pressure last year, you can reduce inoculum size in leaf litter by applying urea and/or lime at this time of the year (fall, but also in spring, or both in spring and fall). The main goal of this practice is to quicken the degradation of leaf litter protecting pseudothecia by microorganisms and worms in and on the soil. Alternatively, when there is no snow cover and it is not muddy, you can use the flail mower to shred the dead leaves to smaller pieces – that will help speed up the degradation of leaf litter by these organisms. In smaller orchards, you can rake the leaves under the trees into the row middles and remove leaf piles with flail mower mode for scalping the sod (Cox, 2016).However, the most used option is to spray apple leaves with urea just 1 to 2 days before the major leaf drop in fall (first severe frost) when leaves are still on the branches. This will secure better coverage of leaves with urea. If you missed to do this you can apply urea to leaf litter on the orchard floor. Use the rate of 40 lbs of urea / acre in 100 gals of water. However, make sure this practice is done late enough in fall and start of cold weather to safely avoid promoting any late growth with the spray applied nitrogen. If you aim to spray leaf litter on the ground, the best timing to do this is late winter but before bud break. Turn the airblast air deflectors downward and/or turn off top nozzles to allow spray mist to lift the loose leaves on the orchard floor and coat them with urea as much as possible. After application, always wash and rinse well your spray equipment since urea can wear up any rubber parts, washers, and gaskets in the sprayer, especially the sprayer pump diaphragm.
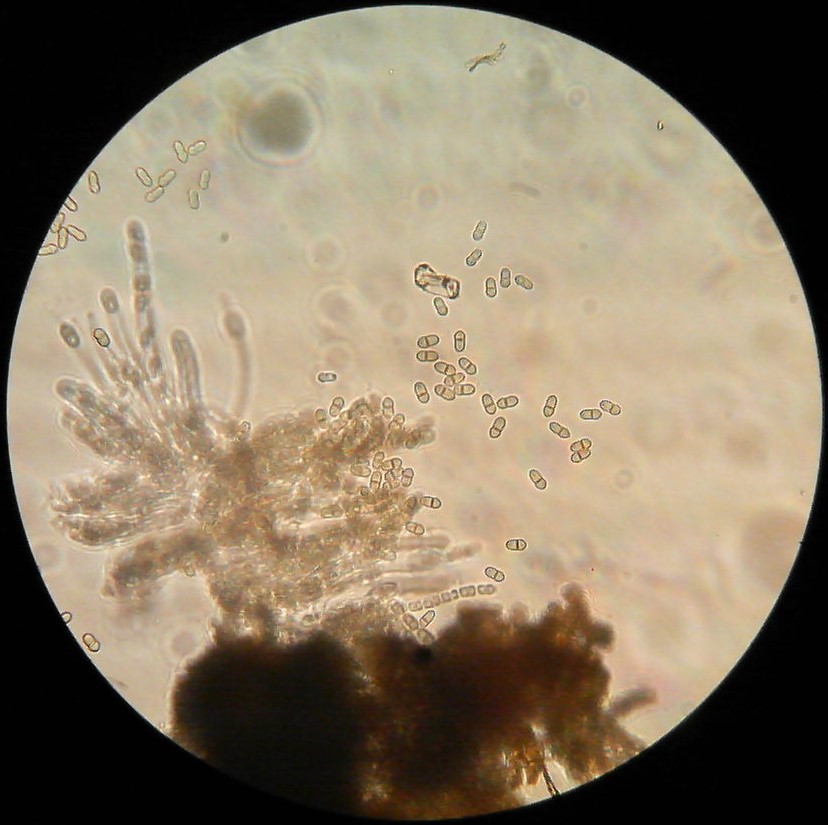
II. MARSSONINA LEAF AND FRUIT BLOTCH (Diplocarpon coronariae)
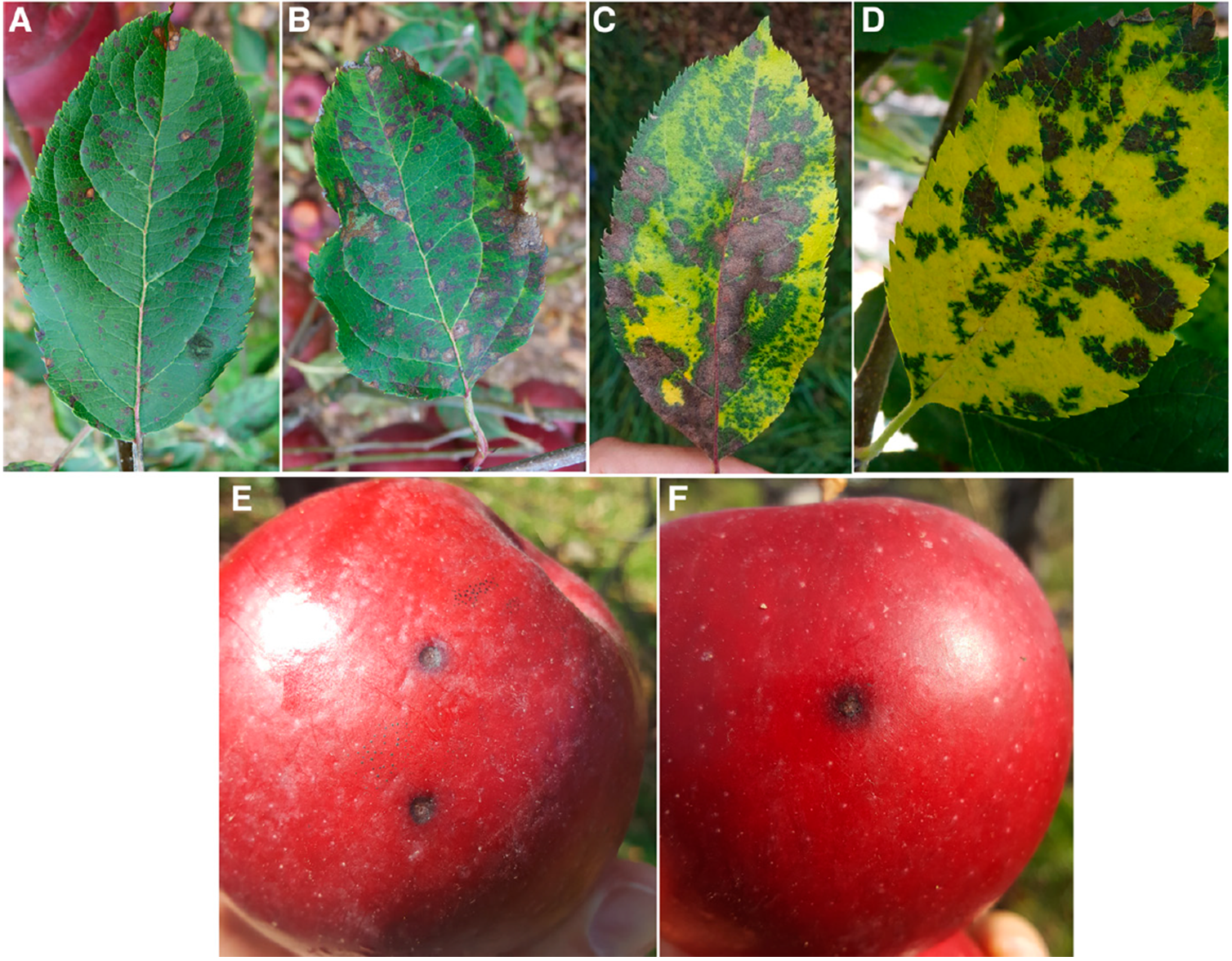
Marssonina leaf blotch (MLB) is an emerging apple disease in Eastern board apple production regions which leads to crown defoliation and fruit spots (Figures 4, 5). It is caused by Diplocarpon coronariae fungus. You can red more about this disease by opening this link: Apple Blotch Disease. MLB defoliation can range between 20 – 70% usually occurring from September to October. Early fall loss of canopy could expose fruit to sun and lead to sunburn, but the added concern are infections of fruit that can happen on trees with significantly defoliated crown and can carry over into storage (we have seen this often in organic orchards). With years of tree crown defoliation, apple trees can get fewer buds to set and weaken due to premature loss of leaf mass to this disease. This reduces accumulation of starch reserves in the trunk and scaffolds and can push more green growth late in the fall when the trees are preparing for winter dormancy. The primary host for D. coronariae is apple (Malus domestica) but other decorative species such as Malus baccata and Chaenomeles spp. are also known to host in this fungus and serve as inoculum sources.

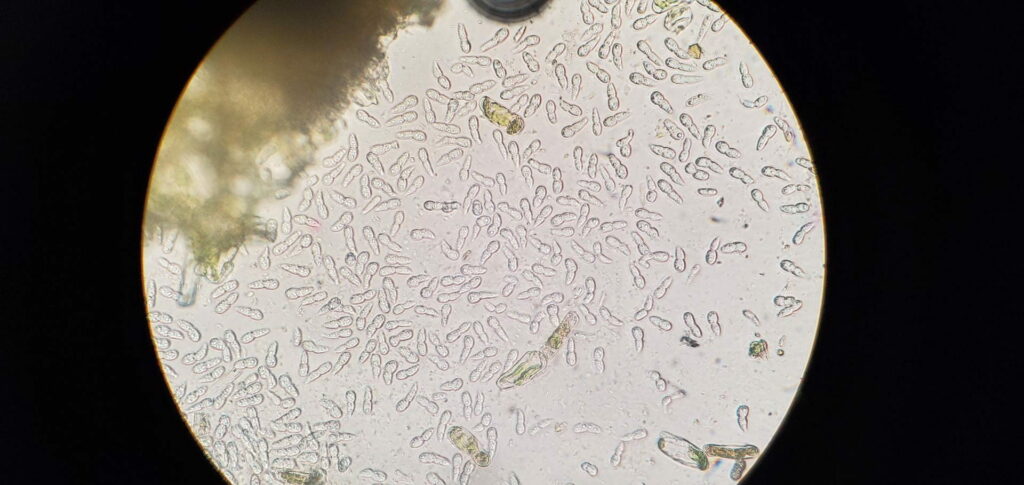
MLB fungus Diplocarpon coronariae overwinters in fallen leaves on the orchard floor (EPPO 2013). Ascospores originating from overwintered fallen leaves cause first infections in spring. They can form in the overwintered cup-like fungal structures called apothecia that form on the leaf litter from last year but it is not known whether the apothecia and Diplocarpon ascospores can form in Virginia climate conditions. It has been reported that in world regions where apothecia do not form the fungus overwinters as asexual spores (conidia) on fallen leaves (Back and Jung 2014). It is highly likely that this is also happening in Virginia. Nevertheless, ascospores still might form and could serve as inoculum for primary infections, while the conidia are asexual spores produced in plate-like bodies on the leaf surface called acervuli (black dots inside the brown blotches which can be seen with a naked eye or a magnifying glass). In the case that ascopores do not form, conidia infect leaves first in mid- to late-spring (Figure 6). Acervuli are spore groups visible as small black round specks after leaf epidermis is ruptured by pushing the spore masses out. Conidia can cause multiple secondary infections during the season. RIMpro: https://rimpro.eu/ offers a disease prediction model that allows to predict when will the first infection of the season occur based on weather forecast and will indicate to you when to start your first spray before the first infection is predicted. It seems that in Eastern US, infections during very rainy summers start late in May or the first week of June. Typical symptoms are usually visible 40 – 45 days after the infection (Lee et al. 2011). In the orchard, spores disseminate by rain and wind. Trade with nursery material that carry infected leaves allows introduction of this disease into the new regions.
The inoculum of MLB can be reduced by orchard sanitation cultural practices. Shredding the fallen leaves on the orchard floor which serve as sources of inoculum, by flail mower or raking and burning the leaves can reduce the spore dose next spring. Application of 40 lbs of urea/A in 100 gals of water onto the overwinter leaves and raking the leaves from under the trees for later shredding by flail mower can reduce the inoculum level of this disease. If urea is not an option (organic orchards) application of dolomitic lime (2.5 tons per acre) just before leaf drop in fall or early in the winter can help apple leaf litter breakdown and thus aids to reduction of the spore inoculum dose in the overwintering leaves (this rate can also be used in apple orchards with synthetic fungicide programs).
III. DORMANT AND SPRING FUNGICIDE SPRAYS FOR LEAF CURL OF PEACH (Taphrina deformans)Copper for leaf curl should be applied while trees are dormant, i.e. wait for the leaves to drop if you want to apply early winter. In most years, due to unpredictability of weather in spring the best time to apply peach leaf curl copper is late in winter and up to and during bud swell (bud break). Use at least 4 to 8 lb of metallic copper per acre. Ziram for peach leaf curl should be used during dormancy, after leaf drop in fall, and prior to bud swell. You can combine Ziram and copper or copper and Bravo (chlorothalonil), but do not apply Ziram and copper during bud swell (bud break). Copper aims to reduce the spores of peach leaf curl fungus Taphrina deformans which overwinter on the tree buds. Infections take place in the spring as the buds open. This fungal pathogen infects buds during rain events from bud swell to bud opening. So if spring is rainy, your application in fall will pay off big time as you will not be able to get into the orchard due to muddy mid-rows. Very long cool wet periods during bud burst can slow peach bud development and thus lead to severe peach curl infections. Copper products also give some suppression of bacterial spot (Xanthomonas arboricola pv. pruni). In spring, if you missed the window for late winter copper application, and you suspect based on cool and wet weather that infection has already occurred, Bravo or Ziram are better than copper as they have efficacy after infection and Bravo and its generics redistribute well during rain.
IV. LITERATURE
Back, C.-G., and Jung, H.-Y. 2014. Biological characterization of Marssonina coronaria infecting apple trees in Korea. Korean J. Mycol. 42:183–190.
Bohr et al. 2018: Symptom occurrence and disease management of Marssonina blotch. 18th International Conference on Organic Fruit-Growing: Proceedings of the Conference, 19-21 February 2018, Hohenheim, Germany.
Cox K. (2016): Having Fungi Yet? Fungicide Update for NY, Scaffolds Vol. 25, No. 1, March 21, 2016, Geneva, NY.
Dang, J. L., Gleason, M. L., Niu, C. K., Liu, X., Guo, Y. Z., Zhang, R., et al. 2016. Effects of Fungicides and Spray Application Interval on Controlling Marssonina Blotch of Apple in the Loess Plateau Region of China. Plant Dis. 101:568–575.
EPPO (2013-103). Diplocarpon mali (anamorph: Marssonina coronaria) – Marssonina blotch of apple. European and Mediterranean Plant Protection Organization, Paris, France.
Lebleu, F. 2015. Chute des feuilles causée par Marssonina – Une menace pour l’arboriculture fruitière biologique/ Falling leaves caused by Marssonina – A threat to organic fruit arboriculture. Arboriculture and special crops FiBL, Switzerland.
Lee et al. 2011: Biological Characterization of Marssonina coronaria Associated with Apple Blotch Disease. Mycobiology. 39: 200–205.
Oberhänsli, T., Vorley, T., Tamm, L., and Schärer, H. J. 2014. Development of a quantitative PCR for improved detection of Marssonina coronaria in field samples. In Proceeding of the 16th International Conference on Organic Fruit-Growing Conference, p. 17–19.
Rosenberger, D. (2005): Jump Starting Apple Scab Control Programs in High-Inoculum Orchards. Available also at: http://www.scaffolds.entomology.cornell.edu/2005/050321.html
Spotts, R. A., Cervantes, L. A., and Niederholzer, F. J. A. 1997. Effect of Dolomitic Lime on Production of Asci and Pseudothecia of Venturia inaequalis and V. pirina. Plant Disease 81:96–98.
Sutton, D. K., MacHardy, W. E. & Lord, W. G. (2000): Effects of Shredding or Treating Apple Leaf Litter with Urea on Ascospore Dose of Venturia inaequalis and Disease Buildup. Plant Disease, 84(12), pp.1319–1326.
Cox K. (2016): Having Fungi Yet? Fungicide Update for NY, Scaffolds Vol. 25, No. 1, March 21, 2016, Geneva, NY.
Agnello et al. (2017): 2017 Cornell Pest Management Guidelines for Commercial Tree Fruit Production. Chapter 6, Disease Management, 6.2.2 Orchard Sanitation for High-inoculum Orchards. Pg. 60.
Ivanović, M. S. & Ivanović, D. M. (2001): Mikoze i Pseudomikoze Biljaka, 2nd ed., Belgrade, Serbia: Poljoprivredni Fakultet, Univerziet u Beogradu, De-eM-Ve, Beograd.
MacHardy, W. E., Gadoury, D. M. & Gessler, C. (2001) Parasitic and Biological Fitness of Venturia inaequalis: Relationship to Disease Management Strategies. Plant Disease, 85(10), pp.1036–1051.
Sutton, D. K., MacHardy, W. E. & Lord, W. G. (2000): Effects of Shredding or Treating Apple Leaf Litter with Urea on Ascospore Dose of Venturia inaequalis and Disease Buildup. Plant Disease, 84(12), pp.1319–1326.
Rosenberger, D. (2005): Jump Starting Apple Scab Control Programs in High-Inoculum Orchards. Available also at: http://www.scaffolds.entomology.cornell.edu/2005/050321.html
